Vertex Announces Primary Endpoint Achieved in Phase 2 Study of VX-864 in Alpha-1 Antitrypsin Deficiency
- Treatment with VX-864 led to a statistically significant increase from baseline in plasma functional AAT levels as compared to placebo and was generally well tolerated -
- Results provide proof-of-mechanism, although magnitude of treatment effect observed unlikely to translate into substantial clinical benefit; VX-864 will not advance into late-stage development -
-
-
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210610005939/en/
“This is the first time that dosing of a small molecule corrector of the Z-AAT protein resulted in significant elevations in both functional and antigenic levels of AAT in people with AATD. We are encouraged by the clear separation of AAT levels in the VX-864 treated groups versus placebo and the favorable safety profile,” said
Efficacy Results
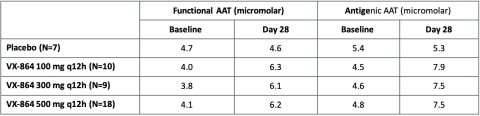
The study met its primary endpoint, with all VX-864 dose groups demonstrating highly statistically significant increases in plasma fAAT levels from baseline compared to placebo at day 28 of treatment. Treatment with VX-864 resulted in a mean increase of 2.2 to 2.3 micromolar in fAAT levels across the three dose groups studied compared to placebo. All dose groups showed a rapid increase in fAAT by day 7 which was sustained over 28 days of treatment. Similar statistically significant increases in antigenic AAT levels were observed compared to placebo, with a mean increase of 2.7 to 3.5 micromolar across the three dose groups studied. Plasma fAAT levels returned to baseline, in the 28-day safety follow-up period following VX-864 discontinuation, consistent with the half-life of native AAT protein and further confirming the biological activity of VX-864.
Figure 1: Statistically significant increase in mean functional and antigenic AAT observed at day 28 compared to placebo

Figure 2: Rapid and sustained increase in mean functional AAT levels from baseline in all dose groups compared to placebo
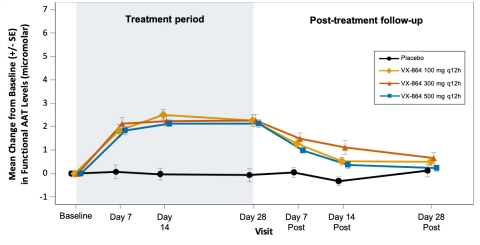
Figure 3: Absolute mean functional and antigenic AAT levels at baseline and at day 28
Safety Results
In this study, VX-864 was generally well tolerated. All but one patient completed treatment. There were no discontinuations due to adverse events (AEs) and there were no serious adverse events (SAEs) considered related to study drug. The majority of AEs were mild or moderate in severity and not treatment limiting. The most common AEs in VX-864 treated patients were diarrhea and nausea. Liver function test (LFT) results were similar between the placebo and VX-864 treated groups, and there was no evidence of any impact on LFT results with VX-864.
Next Steps
The results of the VX-864 study demonstrated proof-of-mechanism with a rapid, consistent and clear effect on functional and antigenic AAT levels and a safety profile consistent with no mechanism-related toxicity. The data collected are anticipated to enable optimization of Vertex’s small molecule corrector approach in AATD and the rapid progression of a portfolio of new molecules with the potential for greater clinical efficacy into the clinic in 2022. In addition, the learnings from the VX-864 study are expected to enable efficiencies in clinical trial design, enrollment and execution for future assets.
About the Phase 2 Study in People with AATD
The Phase 2 study was a randomized, double-blind, placebo-controlled study of the efficacy and safety of VX-864 in people with the PiZZ genotype. People were randomized to one of three dose groups of VX-864 or placebo for 28 days. In addition, there was a 28-day follow-up period after the last dose of treatment. The primary outcome measures were the mean change from baseline in plasma fAAT levels at day 28 compared to placebo as well as safety and tolerability of VX-864.
About Alpha-1 Antitrypsin Deficiency
AATD is a rare, genetic disease characterized by a protein folding defect which can lead to liver and lung disease. AATD is caused by changes in the SERPINA1 gene that encodes the AAT protein. In the most common form of AATD, which occurs in people with a PiZZ genotype, these changes to SERPINA1 cause the body to produce misfolded AAT protein that gets trapped inside the liver, where most AAT is made. This leads to low levels of AAT protein in the blood. Low blood levels of AAT can allow inflammation to proceed unchecked and damage the lungs. The accumulation of defective AAT in the liver can also lead to liver disease. There is currently no cure for AATD. There are also no treatments that target the underlying protein folding defect that is the cause of the disease.
Investor Webcast
The conference call will be webcast live, and a link to the webcast can be accessed on the
Special Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, (i) statements by Dr.
(VRTX-GEN)
View source version on businesswire.com: https://www.businesswire.com/news/home/20210610005939/en/
Investors:
or
or
Media:
mediainfo@vrtx.com
or
or
or
International: +44 20 3204 5275
Source:



