News & Events
Press Release Details
Vertex Announces Positive Results From the VX-548 Phase 3 Program for the Treatment of Moderate-to-Severe Acute Pain
– Treatment with VX-548 led to statistically significant improvement in pain compared to placebo as well as a clinically meaningful reduction in pain from baseline in both the abdominoplasty and bunionectomy randomized controlled trials –
– Treatment with VX-548 was also shown to be effective in the single arm study in a broad range of surgical and non-surgical pain conditions for up to 14 days –
– VX-548 was safe and well tolerated in all three studies –
–
–
Treatment with VX-548 following abdominoplasty or bunionectomy surgery resulted in a statistically significant improvement on the primary endpoint of the time-weighted sum of the pain intensity difference from 0 to 48 hours (SPID48) compared to placebo as well as a clinically meaningful reduction in pain from baseline at 48 hours on the Numeric Pain Rating Scale (NPRS) in both studies (abdominoplasty: LS mean difference in SPID48 between VX-548 and placebo = 48.4 (95% CI: 33.6, 63.1; P<0.0001); bunionectomy: LS mean difference in SPID48 between VX-548 and placebo = 29.3 (95% CI: 14.0, 44.6; P=0.0002)).
For the first key secondary endpoint,
The second key secondary endpoint in both trials was time to meaningful pain relief defined as ≥2-point reduction in NPRS from baseline compared to placebo. VX-548 had a more rapid onset to meaningful pain relief than placebo in both the abdominoplasty and bunionectomy trials. (The median time to meaningful pain relief was 8 hours for placebo in both studies compared to 2 hours in abdominoplasty and 4 hours in bunionectomy for VX-548, with nominal P<0.0001 and 0.0016, respectively.)
Other secondary endpoints in both trials were generally consistent with the primary endpoint.
The Phase 3 single arm safety and effectiveness study evaluated treatment with VX-548 for up to 14 days across a broad range of other surgical and non-surgical acute pain conditions and demonstrated favorable safety and tolerability, as well as effectiveness as measured by a Patient Global Assessment (PGA) at the end of treatment (83.2% of patients rated VX-548 as good, very good, or excellent in treating pain).
VX-548 was safe and well tolerated in all three Phase 3 studies. The majority of adverse events (AEs) were mild to moderate, and there were no serious adverse events (SAEs) related to VX-548. In general, AEs in the two randomized controlled trials were consistent with the post-surgical setting. In the VX-548 arm, the incidence of AEs was lower than placebo (patients with any AEs in VX-548 and placebo arms: 50.0% and 56.3%, respectively, following abdominoplasty, and 31.0% and 35.2%, respectively, following bunionectomy).
“We are very pleased with the results from the VX-548 pivotal program, which demonstrate a compelling and consistent combination of efficacy and safety across multiple acute pain conditions and settings. The VX-548 benefit-risk profile ideally positions it to potentially fill the gap between medicines with good tolerability but limited efficacy and opioid medicines with therapeutic efficacy but known risks, including addictive potential,” said
“As a physician treating patients suffering from pain for many years, I know firsthand the critical need for new, efficacious and safe treatment options,” said
VX-548 Phase 3 Results in Patients Undergoing Abdominoplasty
Efficacy Results
Patients aged 18 to 80 years with moderate or severe pain after abdominoplasty surgery were eligible to participate in the trial. 1,118 patients were randomized and dosed with either VX-548 administered orally with an initial dose of 100 mg followed by 50 mg every 12 hours (at 12, 24 and 36 hours after the first dose), hydrocodone bitartrate/acetaminophen (5 mg/325 mg administered orally every 6 hours over 42 hours), or placebo.
Primary and Key Secondary Outcomes in Phase 3 Study of Acute Pain Following Abdominoplasty
|
Placebo |
HB/APAP |
VX-548 |
|
|
Primary endpoint of SPID48 vs. placebo |
|
|
|
|
LS mean SPID48 (SE) |
70.1 (6.1) |
-- |
118.4 (4.3) |
|
LS mean SPID48 difference from placebo |
-- |
-- |
48.4 |
|
95% CI |
-- |
-- |
(33.6, 63.1) |
|
P value vs. placebo |
-- |
-- |
<0.0001 |
|
First key secondary endpoint of SPID48 vs. HB/APAP |
|
|
|
|
LS mean SPID48 (SE) |
-- |
111.8 (4.3) |
118.4 (4.3) |
|
LS mean SPID48 difference from HB/APAP |
-- |
-- |
6.6 |
|
95% CI |
-- |
-- |
(-5.4, 18.7) |
|
P value vs. HB/APAP |
-- |
-- |
0.2781 |
|
Second key secondary endpoint of time to ≥2-point reduction in NPRS from baseline vs. placebo (minutes) |
|
|
|
|
Median time |
480 |
-- |
119 |
|
95% CI |
(477, 705) |
-- |
(90, 180) |
|
P value* vs. placebo (Log-rank) |
-- |
-- |
<0.0001 |
|
*P value is nominal; CI = confidence interval; LS = least squares; SE = standard error. |
|||
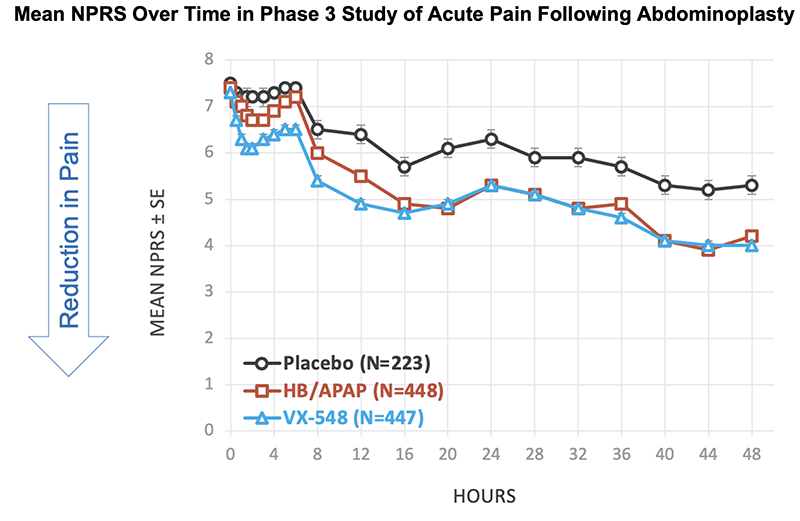
NPRS Reductions From Baseline at 48 Hours in Phase 3 Study of Acute Pain Following Abdominoplasty
|
Placebo |
HB/APAP |
VX-548 |
||
|
Baseline NPRS, mean |
7.5 |
7.4 |
7.3 |
|
|
Change from baseline in NPRS at 48 hours, mean |
-2.3 |
-3.2 |
-3.4 |
|
|
% reduction from baseline in mean NPRS at 48 hours |
31% |
43% |
47% |
Safety Results
VX-548 was generally well tolerated in this study. The majority of adverse events (AEs) were mild to moderate, and there were no serious adverse events (SAEs) related to VX-548.
In general, AEs were consistent with the post-surgical setting. In the VX-548 arm, the incidence of AEs was lower than placebo (patients with any AEs in VX-548 and placebo arms: 50.0% and 56.3%, respectively).
AEs with an incidence ≥5% in any treatment groups (VX-548, placebo, or HB/APAP, respectively) were nausea (19.0%, 25.2%, 32.8%), constipation (10.5%, 10.8%, 8.7%), headache (4.2%, 5.0%, 7.1%), dizziness (4.0%, 7.7%, 5.4%), and hypotension (2.5%, 6.8%, 3.6%).
VX-548 Phase 3 Results in Patients Undergoing Bunionectomy
Efficacy Results
Patients aged 18 to 80 years with moderate or severe pain after bunionectomy surgery were eligible to participate in the trial. 1,073 patients were randomized and dosed with either VX-548 administered orally with an initial dose of 100 mg followed by 50 mg every 12 hours (at 12, 24 and 36 hours after the first dose), hydrocodone bitartrate/acetaminophen (5 mg/325 mg administered orally every 6 hours over 42 hours) or placebo.
Primary and Key Secondary Outcomes in Phase 3 Study of Acute Pain Following Bunionectomy
|
Placebo |
HB/APAP |
VX-548 |
|
|
Primary endpoint of SPID48 vs. placebo |
|
|
|
|
LS mean SPID48 (SE) |
70.6 (6.3) |
-- |
99.9 (4.5) |
|
LS mean SPID48 difference from placebo |
-- |
-- |
29.3 |
|
95% CI |
-- |
-- |
(14.0, 44.6) |
|
P value vs. placebo |
-- |
-- |
0.0002 |
|
First key secondary endpoint of SPID48 vs. HB/APAP |
|
|
|
|
LS mean SPID48 (SE) |
-- |
120.1 (4.5) |
99.9 (4.5) |
|
LS mean SPID48 difference from HB/APAP |
-- |
-- |
-20.2 |
|
95% CI |
-- |
-- |
(-32.7, -7.7) |
|
P value vs. HB/APAP |
-- |
-- |
0.0016 |
|
Second key secondary endpoint of time to ≥2-point reduction in NPRS from baseline vs. placebo (minutes) |
|
|
|
|
Median time |
480 |
-- |
240 |
|
95% CI |
(476, 716) |
-- |
(117, 477) |
|
P value* vs. placebo (Log-rank) |
-- |
-- |
0.0016 |
|
*P value is nominal; CI = confidence interval; LS = least squares; SE = standard error. |
|||
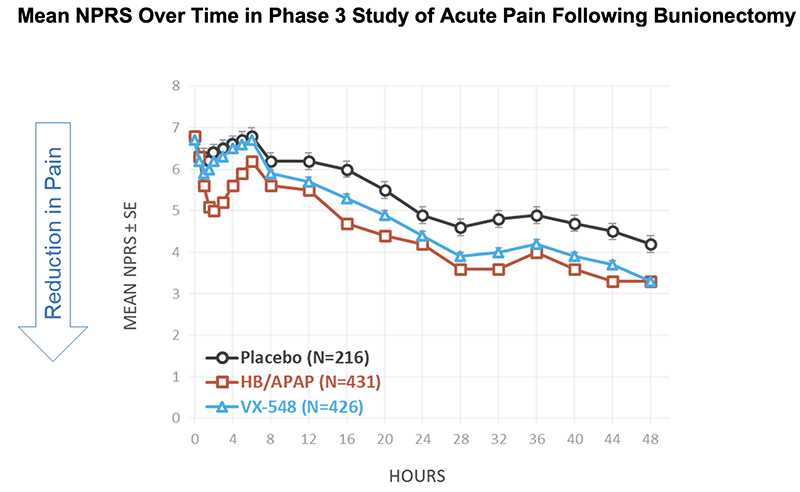
NPRS Reductions From Baseline at 48 Hours in Phase 3 Study of Acute Pain Following Bunionectomy
|
Placebo |
HB/APAP |
VX-548 |
||
|
Baseline NPRS, mean |
6.8 |
6.8 |
6.7 |
|
|
Change from baseline in NPRS at 48 hours, mean |
-2.6 |
-3.6 |
-3.4 |
|
|
% reduction from baseline in mean NPRS at 48 hours |
38% |
53% |
51% |
Safety Results
VX-548 was generally well tolerated in this study. The majority of AEs were mild to moderate, and there were no SAEs.
In general, AEs were consistent with the post-surgical setting. In the VX-548 arm, the incidence of AEs was lower than placebo (patients with any AEs in VX-548 and placebo arms: 31.0% and 35.2%, respectively).
AEs with an incidence ≥5% in any treatment groups (VX-548, placebo, or HB/APAP, respectively) were nausea (8.2%, 10.6%, 14.4%), headache (4.9%, 9.3%, 10.4%), constipation (3.5%, 4.2%, 5.1%), and dizziness (3.5%, 5.1%, 5.3%).
VX-548 Phase 3 Safety and Effectiveness Study Results
VX-548 was generally safe and well tolerated in this study. AEs were mostly mild or moderate, and there were no SAEs related to VX-548. The safety profile in this study was generally consistent with randomized, controlled Phase 3 studies with VX-548.
Patient perception of VX-548 effectiveness in treating pain as measured by a patient global assessment (PGA) at the end of treatment showed 83.2% of patients reporting VX-548 as good, very good, or excellent.
Next Steps for the Pain Portfolio
In addition,
In line with its portfolio strategy,
Conference Call and Webcast
The company will host a conference call and webcast at
The conference call will be webcast live and a link to the webcast can be accessed through
About Acute Pain
Defined as pain lasting <3 months, acute pain is a disabling condition that affects over 80 million people in the
About VX-548
VX-548 is an investigational oral, selective NaV1.8 pain signal inhibitor that is highly selective for NaV1.8 relative to other NaV channels. NaV1.8 is a voltage-gated sodium channel that plays a critical role in pain signaling in the peripheral nervous system. NaV1.8 is a genetically validated target for the treatment of pain, and
About
Special Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements by
(VRTX-GEN)
View source version on businesswire.com: https://www.businesswire.com/news/home/20240129843577/en/
Investors:
InvestorInfo@vrtx.com
Media:
mediainfo@vrtx.com
or
or
International: +44 20 3204 5275
Source:



